Compliance isn’t optional in pharma sales — it’s critical. One misstep in recording a healthcare professional (HCP) interaction can lead to major fines or reputational damage. As regulations tighten globally, CRM for pharma sales reps has become essential—not just for improving productivity, but for ensuring compliant communication at every step.
According to a Deloitte study, over 60% of life sciences companies invest in CRM systems specifically for compliance tracking and field force effectiveness.
In this blog, we’ll explore how CRM solutions tailored for pharma help reps manage field activities, document HCP interactions, and remain compliant — all while boosting sales outcomes.
Business Need & Importance
Why Compliance Is the Cornerstone of Pharma Sales
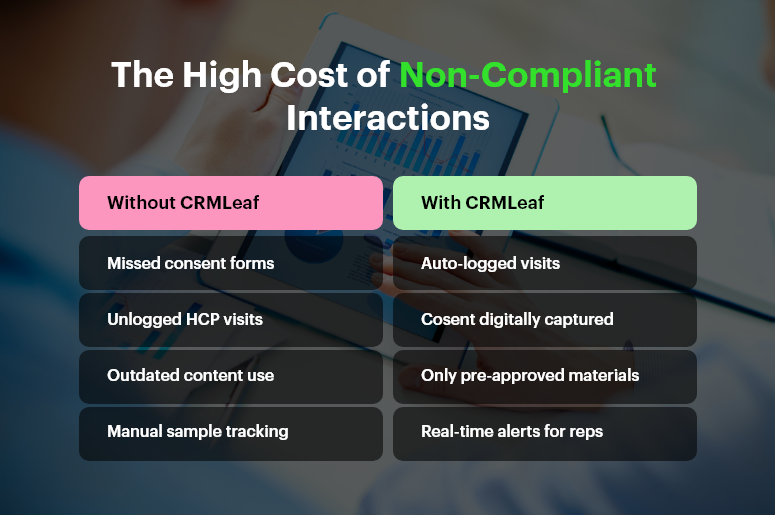
Pharmaceutical sales teams operate under strict scrutiny. From FDA and HIPAA in the U.S. to global codes like EFPIA, regulations demand that every interaction with doctors, hospitals, or pharmacists is tracked, transparent, and auditable.
Missing even a single compliance checkpoint — such as forgetting to log a visit, sharing off-label product info, or failing to record expenses properly — can lead to legal risks or corporate penalties.
For small and mid-sized pharma companies especially, this challenge is magnified by:
- Decentralized data entry methods like Excel sheets, paper forms, or separate apps.
- Limited oversight into on-field sales reps’ activities.
- Growing complexity of multi-region compliance laws.
Industries That Depend on This
- Pharmaceuticals & Life Sciences: Where HCP interactions and promotional activities must follow strict compliance documentation.
- Medical Device Sales: Where reps often demonstrate products on-site and must log usage and feedback accurately.
- Healthcare Software Providers: Where CRM must track demos, onboarding, and support interactions with hospitals and clinics.
A CRM for pharma sales reps isn’t just a sales tool — it’s a compliance framework in action.
Best Practices & Actionable Tips
How to Ensure Compliant HCP Interactions Using a CRM
A modern CRM for pharma sales reps ensures every field activity is logged, monitored, and compliant. Here’s how to put it into practice:
🔹 Standardize HCP Interaction Logging
Ensure every call, visit, or digital touchpoint is recorded within the CRM. Reps can log visit outcomes, topics discussed, and follow-up actions in real time — reducing the risk of missed data or non-compliant exchanges.
🔹 Track Sample Distribution & Promotional Materials
Capture all product samples, brochures, and informational materials given to HCPs. CRMLeaf enables reps to select approved materials, log distribution details, and even attach digital acknowledgments — helping companies meet transparency regulations.
🔹 Set Access Controls Based on Role
Limit what reps can access and share based on compliance guidelines. Reps can only send approved documents, while managers have visibility into audit logs, ensuring materials align with regulatory boundaries.
🔹 Automate Expense Reporting with Audit Trails
Let reps record travel, meals, or sponsorship expenses with receipts attached. These entries feed into CRMLeaf’s backend reporting for finance and compliance teams — ensuring full traceability and audit readiness.
🔹 Enable GPS-Stamped Activity Logs
Use geo-tagging to verify field rep visits to clinics, pharmacies, or hospitals. This not only increases accountability but also prevents data tampering or post-entry manipulation.
🔹 Integrate CRM with Medical Databases
Link CRM to databases of HCPs, product libraries, and compliance rules. This ensures reps only interact with licensed professionals and distribute the correct drug information.
🔹 Enable Offline Access for Field Work
Reps in remote locations can log visits offline, and the CRM syncs once connected. No excuse for missed records or late data uploads — compliance stays intact even without constant internet access.
🔹 Trigger Compliance Alerts and Approvals
Set up real-time alerts for non-compliant activities. For example, flagging if a rep tries to schedule multiple visits to the same HCP in a restricted period or enters unsupported product claims.
🔹 Store Consent Records and Digital Signatures
Collect e-consent for samples, information sharing, or data usage. CRMLeaf’s digital consent logs serve as a legal backup in audits or disputes.
🔹 Link CRM with ERP for Expense and Logistics Control
With CRMLeaf’s CRM + ERP integration, expenses, inventory, and sample stock sync seamlessly. This enables end-to-end compliance tracking from budget to delivery.
Customer Success
For example, MedNova Biotech, a mid-sized pharma company with 50+ field reps, adopted CRMLeaf’s CRM for pharma sales reps to simplify their compliance workflows.
Before CRMLeaf, the team used spreadsheets and email logs to record visits — resulting in missed entries and compliance flags during audits.
After switching to CRMLeaf:
- Compliance violations dropped by 70% within 6 months
- Sample tracking improved accuracy by 85%
- Audit preparation time was reduced from 5 days to just 1 day
CRMLeaf provided MedNova with a centralized platform to log HCP visits, control document sharing, and keep accurate expense records — all in one place.
Key Takeaways & Closing
A dedicated CRM for pharma sales reps goes far beyond contact management — it’s your frontline defense against non-compliance.
With CRMLeaf, your sales team can:
- Document every HCP interaction in real time
- Distribute approved materials only
- Track samples, expenses, and visits with zero manual errors
In an industry where compliance and trust are everything, CRMLeaf helps your team sell confidently — without compromising regulations.


